For years, Alzheimer's conferences were like the obituary pages in the local newspaper: It's where clinicians and researchers in the field went to find out the names of the latest promising drugs to die. Between 1998 and 2017 alone, 146 clinical trials of new Alzheimer's drugs failed.
So when Randall Bateman showed up outside a restaurant in Bar Harbor, Maine, one evening in the fall of 2022 during an industry confab and announced to a couple of tables full of his colleagues drinking on the patio that he had something important to share with them, no one was prepared for what came next.
Bateman, a neurologist and Alzheimer's researcher at the Washington University School of Medicine in St. Louis, told them he had just received a phone call from his contact at the drug company running the trials of lecanemab, an experimental drug designed to facilitate the removal of the toxic plaques in the brain associated with the disease. The results, set for public release the following morning, were in: In a study of 1,800 Alzheimer's patients over 18 months, the treatment had reduced the rate of cognitive decline by close to 30 percent.

There was a stunned silence and some skeptical questions. Then the table erupted in applause.
"It's all anybody talked about for the rest of the meeting," says Bateman. "The agenda was blown. Nobody was paying attention to what we should have been doing. It's reenergized, recharged, everything."
The trial results Bateman reported was just the first in a flurry of positive developments. A few months later, the U.S. Food and Drug Administration approved the drug, now marketed as Leqembi. In July, Eli Lilly reported that a second drug, donanemab, had an even greater effect on slowing the progress of the disease, in a study of more than 1,700 Alzheimer's patients. The FDA is expected to approve it soon. As with Leqembi, many expect that Medicare will eventually agree to pay for it—marking the first time new treatments have become available for the terminal neurodegenerative disease in 20 years.
To be sure, these drugs are not cures. They offer little relief for patients already in the late stages of Alzheimer's dementia, for whom the effects of the new drugs are modest. They can slow the decline, giving those in whom the disease is moderately advanced an extra year of cognitive lucidity. For those patients and their families that's certainly significant, but nowhere close to a reversal.
The big potential benefit, say Alzheimer's experts, lies in using these drugs, or others soon to come, in conjunction with a second recent development in the field: diagnostic blood tests that can identify the presence of Alzheimer's-associated proteins. With more many blood tests currently undergoing clinical trials, scientists believe that in a few years clinicians may be able to use them to make quick, early diagnoses cheaply, even before patients show any outward symptoms of dementia. That suggests a new strategy against the disease: GPs could screen otherwise healthy people for early-stage Alzheimer's and treat them with drugs that slow the progress of the disease before major damage has occurred. The hope is that eventually, Alzheimer's will no longer be a terminal disease but a chronic one that can be managed with drugs and perhaps be staved off indefinitely.
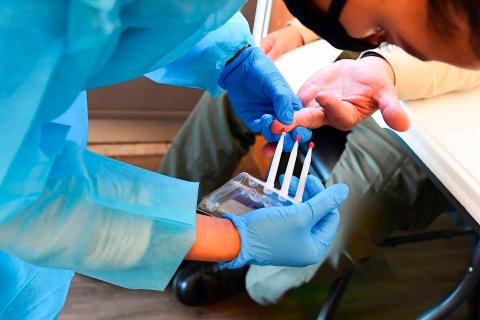
There is no guarantee, however, that the drugs and tests now being developedwill fulfill this promise. Some scientists, skeptical of the influence of Big Pharma, believe the field is moving too quickly, particularly in starting clinical trials on patients who have no symptoms of dementia. And even with the best drugs and tests, many hurdles would still remain before early detection and prevention could become a standard of care—not least the U.S. health care system's abysmal track record in preventing chronic, slow-developing diseases, such as diabetes and heart disease, that are already eminently manageable in theory.
Still, many Alzheimer's researchers are calling the new developments, which break a 20-year logjam in Alzheimer's research, a "game changer." That is hopeful news for the 13 million Americans who are expected to have the disease by 2050 (up from 11 million today), and millions of their loved ones.
Plaques and Tangles
When Randall Bateman was still a medical student back in 1990s, researchers and the clinicians who treated patients were basically flying blind. The only way to know for sure if a patient or research subject suffering from dementia had Alzheimer's Disease, and not another kind of dementia, was to wait for them to die and then examine a slice of their brain tissue under a microscope.
Diagnosis, in other words, was confirmed using the same crude methods available to Alois Alzheimer, the German psychiatrist and pathologist who first discovered the disease in 1906: by the observation of cognitive decline, followed by postmortem visual identification of the presence of dark clumps of what were then known as "senile plaques," along with stringy protein molecules, "neurofibrillary tangles," that clogged up the spaces between the neurons. (Alzheimer also noted massive cerebral shrinkage, caused by the death of brain cells, another hallmark of the disease).
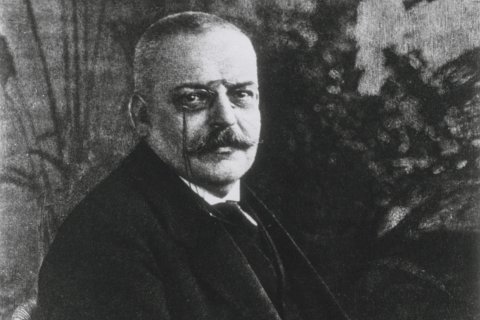
Although there had been some significant advances since Alzheimer's time, up until the early 2000s, the medical field's understanding of the disease was still relatively basic. In the 1980s, scientists determined that the plaques were cellular garbage, consisting of clumps of a protein called amyloid beta, or a-beta, a naturally occurring byproduct of normal brain activity (the function of which is not well understood). In healthy individuals a-beta is regularly cleared from the brain along with other metabolic waste, particularly during sleep. The tangles are made of "tau," knots of insoluble protein filaments normally involved in maintaining the structural stability of the microscopic tubules that allow the transportation of materials throughout cells.
In the years that followed, the cause of Alzheimer's disease was hotly debated, with much of the field split between two competing camps, engaged in what was in many ways still a speculative argument they did not yet have the tools to resolve. The "Baptists" believed it was the accumulation of the beta amyloid plagues that kicked off the deadly cascade that led to everything else. The "Taoists" suspected that tau was to blame. Scientists on both sides usually had a personal stake—they chose their area of research based on which hypothesis they believed.
By the late 1990s, what would become known as the "amyloid cascade hypothesis" was beginning to become the field's dominant paradigm, thanks to the discovery of families carrying rare mutations that caused them to develop the symptoms of full-blown Alzheimer's disease far earlier than usual. With new genetic tools of the time, scientists showed that most of the individuals in these families who developed early-onset Alzheimer's carried mutations that could be directly linked to the buildup of the amyloid-beta plaques. That evidence helped catalyze an explosion in drug trials, virtually all of which targeted the removal of the plaques from the brain. Until recently, all of the amyloid-beta drugs failed.
The recent success of lecanemab has given Baptists fresh ammunition. They argue that the finding that showed that removing amyloid-beta slowed the disease progression is a "proof of principal" that the amyloid plaques are what drive it. But even amyloid diehards admit that the old labels no longer fit. The disease seems to be far more complex than anything contained in the old theories of Baptists or Taoists. Even if amyloid-beta is the "kindling that lights the fire," once that fire starts the brain heads toward a systemic collapse that involves far more than the buildup of plaque. Paul Aisen, director of the Alzheimer's Therapeutic Research Institute (ATRI) at Keck School of Medicine of USC and a leading expert on clinical trial design, makes no apology for his faith in the a-beta-cascade hypothesis—indeed, he claims his faith never wavered.
Still, he attributes the field's long string of failures to an early misunderstanding of the progression of what was then a relatively new disease, and the technologies available prior to the early 2000s. "Amyloid could only be seen at autopsy, so we would develop drugs that worked in a test tube or a mouse and test them in humans and they wouldn't work, and we wouldn't know why," says Aisen. "It interferes with a rational drug development plan when you can't see what you're doing."
That began to change in in 2004, with the debut of Positron emission tomography, or PET, an imaging technique that allowed clinicians to peer inside the brains of living patients. For the first time, they could get images of amyloid and, soon after, tau in the brains of living Alzheimer's proteins.

The impact was immediate and profound. Suddenly scientists could scan the brains of the patients through drug trials and visually track the impact of drugs over time. They could clearly see, for instance, that some treatments seemed to have no effect, while others, such as certain types of antibodies that directly attacked the plaques, were clearly helping to remove plaques from the brain. For the first time they could adjust the doses up and down, rather than just guessing.
PET imaging also allowed scientists to address another powerful confounding factor: the inclusion of a significant number of individuals in Alzheimer's trials who had been misdiagnosed. We now know that the problem was so widespread that it alone could have torpedoed many of the early amyloid-beta trials.
The evidence comes from a massive, multi-site, multi-year study published in 2019, in which 946 dementia experts administered PET scans to more than 11,000 Medicare recipients who had already been diagnosed with dementia or mild cognitive impairment. They found, remarkably, that 35.6 percent, or about a third of patients, had either been incorrectly diagnosed with Alzheimer's disease or actually had Alzheimer's but had been misdiagnosed with another condition, such as frontotemporal dementia, which in its observable clinical manifestations often appears identical to those seen in people with Alzheimer's.
Aisen characterizes the slowdown in the progression of the disease seen in the lecanemab trial as "really big." He attributes the outcome directly to the ability of researchers to evaluate experimental drugs effectively using imaging and make adjustments to dosing in real time.
"Being able to see what we were doing is what is leading to success," he says. "It's now clear—for the first time, really, with lecanemab—that we can slow the disease down. People should be taking this drug."
Perhaps most important for future research, he says, the advent of brain imaging and other technological innovations have allowed researchers to precisely track how the brain changes as the disease develops. This has forced the field to confront an unpleasant truth: Even if earlier drugs had been effective, they were likely administered way too late to significantly alter the course of the disease. They were, in other words, destined to fail. This, he argues, also explains why lecanemab can slow the progression of those in the initial trials but is powerless to halt it.
The evidence comes from the results of a two-decade-long "observational" longitudinal study Aisen has helped coordinate called the Alzheimer's Disease Neuroimaging Initiative (ADNI), which has fundamentally changed our understanding of the disease. Researchers used imaging, genetics and cognitive, clinical and functional tests, as well as other tools, to painstakingly track and document changes in the brains of more than 2,500 people as the disease progressed. The goal was to "deeply characterize" and "map out the disease from start to finish."
When Aisen started, Alzheimer's disease was thought to begin with the onset of observable cognitive and functional decline and, after progressing for seven years or so, result in death. But the onset of early dementia, we now know, is in fact just the final phase in a far longer progression that takes place over roughly 25 years. It begins with the accumulation of amyloid-beta at least 18 years before any behavioral changes are detectable to most future patients or their loved ones.
This realization came as a rude awakening for clinicians on the front lines of the epidemic who have worked closely with patients and their loved ones for decades. Administering drugs that target amyloid in patients who already have noticeable memory impairment is akin to treating someone suffering from congestive heart failure with statins in the hopes of lowering their cholesterol, says Rudolph Tanzi, professor of neurology at Harvard University. Or, as Eric Reiman, a neuroscientist at the Banner Alzheimer's Institute in Phoenix, puts it, treating a tumor in a patient whose cancer is already metastatic.
"Hitting amyloid in a patient that already has Alzheimer's disease isn't going to make them better," says Tanzi, a pioneer in the field who, in the 1980s, was among the first to identify the genes associated with the disease. "We don't diagnose Alzheimer's disease until the brain is already deteriorated to the point of dysfunction in the form of memory impairment. Even in what we've traditionally considered the earliest stages of Alzheimer's disease, where the symptoms are just beginning, the brain is already full of amyloid and there's already enough inflammation in the brain and synapse loss to start leading to cognitive dysfunction."
Quick Progress
One reason Alzheimer's specialists are so excited about the recent developments is that the field had been stagnating for two decades. Drug companies spent $600 billion on developing Alzheimer's treatments that failed. The National Institutes of Health handed out billions of dollars for research that yielded no significant benefit to patients. The early failures damaged the reputation of medical research establishments and the pharmaceutical companies.

The FDA made things worse when, in 2021, it overruled an outside panel of experts to approve aducanumab (brand name: Aduhelm), a drug that attacks amyloid plaque and was shown to have a modest benefit for patients. Some researchers suggested that the FDA approved the drug simply to keep big pharmaceutical companies from giving up and walking away from Alzheimer's research altogether. Biogen initially suggested the drug would come with a price tag of roughly $56,000 a year, a bill many considered outrageous. In the wake of the decision, three of the FDA advisers on the overruled panel resigned, lawmakers in Washington launched congressional investigations and the Centers for Medicare & Medicaid Services (CMS), the government agency that oversees Medicare, declined to pay for the drug.
For this reason, many people, including some scientists, are deeply skeptical of any good news coming from the FDA and Big Pharma. "There's a huge financial incentive involved here, because the FDA approval of these drugs means billions of dollars in profits for the pharmaceutical companies," says Karl Herrup, a professor of neurobiology and an investigator in the Alzheimer's Disease Research Center at the University of Pittsburgh School of Medicine He suggested the FDA's approval was suspect. "I think that's driving them."
Herrup, author of a book titled How Not to Study a Disease: The Story of Alzheimer's, suggests it was the "vested position" of many of the field's most well-known scientific figures that handcuffed progress for so long. These researchers have "spent so much time and energy and reputation pushing the concept of amyloid as a driver of Alzheimer's that it's really hard to admit that it doesn't work. My feeling is that the focus on a-beta has totally distorted even our definition of the disease. We've been chasing rainbows because of that."
This time, at least, Herrup's view seems to be in the minority. The FDA advisory board unanimously endorsed the approval of Leqembi. This and other new drugs in the pipeline, some scientists argue, herald the beginning of new era driven by the development of increasingly powerful diagnostic tools that facilitated a more sophisticated understanding of how the disease unfolds.
Learning lessons from the aducanumab fiasco, Biogen and its Tokyo-based partner Eisai, the makers of both aducanumab and the new drug lecanemab, made sure their trial design, and the outcomes that would indicate success, were less ambiguous, easier to understand and more likely to satisfy the scientific community. After the drug won approval, the firms announced plans to price it at $26,500—higher than the $8,900 to $21,500 the independent Institute for Clinical and Economic Review (ICER) estimates it's worth, but close enough that CMS is expected to cover it for as many as a million patients—about 90 percent of the market. ICER estimates the cost per patient would come to $82,500 per year.
Despite the drug's limitations, the lecanemab trials that led to its recent FDA approval, many scientists argue, is still an important first step: now that the field has its first drug that has been proven effective in removing amyloid, it has a real shot at earlier intervention. To Aisen, in fact, the limited effect the drugs had in late stage patients is, in fact, remarkable. In the 25-year arc of Alzheimer's disease, Aisen says, the participants in the lecanemab trial roughly fell in years 13 to 20. By then, the plaques have created irreversible damage. They have killed neurons and catalyzed a spiral of negative effects, including the development of tau tangles and the accumulation of other proteins in the brain. Vascular changes have also occurred, choking off the healthy delivery of nutrients and essential signaling agents. The brain is inflamed. Microglia—immune cells in the brain—have begun to target areas that are in crisis, the same way they would target areas of the brain that have become infected, by moving in and beginning to gobble them up in a misguided effort to arrest the spread of dysfunction to the rest of the vital organ. Amid this raging neurological forest fire, the fact that the new drugs hold at bay the rapid cognitive and functional decline seen in other patients for 12 months is, to Aisen, a testament to its strength.

In the hopes of further arresting this systemic collapse, Aisen is co-leading the AHEAD trial, which is in the midst of recruiting "presymptomatic" patients to participate in a four-year study of lecanemab. The new cohort will consist entirely of people who are, for the most part, cognitively and functionally asymptomatic but have abnormal levels of amyloid in their brains. The idea is to capture people in years one through 10 of the progression. A later phase of the trial, he says, will target a cohort who is "even closer to year one."
At the same time, the TRAILBLAZER study, co-led by Banner Institute's Reiman, is taking a similar approach, but using donanemab, the new drug from Eli Lilly that is expected soon to win FDA approval, to target those in earlier stages of the disease.
Both studies will also use imaging tests that are now capable of detecting and measuring tau tangles, along with blood tests that will do the same, to see if they can arrest that part of the disease. Even at this early stage of the disease, however, Aisen and Reiman both say the new drugs alone will likely still not be sufficient to halt the progress of the disease. For that, Aisen believes it will be necessary to add anti-tau drugs that go after tangles, too. To that end, Aisen and his collaborators are setting up another trial that will also use experimental anti-tau drugs to remove any tangles that accumulate.
It will take five years before AHEAD yields results, estimates Aisen, and 10 years before we have a real handle on how to prevent the disease. Reiman is slightly more optimistic but agrees many more steps are needed.
Still, both see a bright future ahead.
"Lecanemab is a real landmark. Having a fully approved drug [that] slows the disease is significant. But we've got to move on and increase the benefit," Aisen says. "I'm very optimistic. We're just going to build on this success. Things will get better and better until the entire population is routinely screened for risk and the risk is fixed before the disease starts."
Not everybody shares their optimism. Some worry that the field is moving too fast. Madhav Thambisetty, a senior investigator in the NIH's Intramural Research on Aging and a practicing neurologist who treats dementia patients at the Johns Hopkins Memory and Alzheimer's treatment center, says he has urged caution when referring interested patients in the early stages of the disease for trials.
He calls the clinical benefits "small to modest," and "marginal at best," but said they are "very exciting" for researchers because it's the first time the field has drugs that can precisely target one of the pathological features of the disease.
"The clinical meaningfulness of these results remains to be established, but the fact that they do have a clinical benefit that's statistically significant [is] very clear," he says.
Still, he worries the results have led to a "lack of balance in the way the narratives have built around the new treatments." Much of what has been reported about the new class of drugs, he says, has centered on the debates in the field over whether the clinical effects seen on patient cognition and function are meaningful to clinicians and caregivers. Of more concern for his patients, he says, are the potential side effects and "adverse events" associated with the drugs, which he believes have been largely overshadowed by the hype.
He notes that the anti-amyloid drugs as a class have received a black box warning from the FDA, the highest level of warning for drugs, one reserved for those that are associated with the risk of serious harm and even death. The warnings come in response to swelling in the brain and microbleeds sometimes associated with the medication. Much of the commentary, he says, has noted that these symptoms are mostly mild, can be detected on MRI scans and managed acutely. But this assessment is based on incomplete information, he says. The impact these side effects had on cognition in the months that follow treatment, he says, has not been publicly reported. Because of this "lack of transparency," there is no way to know whether the brain bleeds and swelling seen in patients eventually accelerates the cognitive and functional decline that the drug claims to slow.
"To me, a measure of the effect at 18 months, which is the end of the trial, [is] avery basic metric of assessing risk with these drugs that really has not been reported out," he says. "Brain bleeding and brain hemorrhages [are] not benign. So, unless I see evidence that they're not related to adverse clinical outcomes in the months that follow the initial reports, my worry is that they are. I don't think we've learned enough from these trials to move on to the next phase, which is potentially testing these in asymptomatic individuals for as long as four years, which is longer than in earlier phases."
Some of those involved in the new trials have downplayed the concerns. Reiman, in fact, believes these side effects might be less of an issue for those in an earlier phase of the disease. In the late stages, he says, "you're having to pull amyloid plaque out of the brain, but also off of blood vessels, which is the main reason for the side effects," such as bleeding and swelling, he says. In the brains of those who are at earlier stages of the disease, the plaques "haven't been there as long. So, they're less adherent, and you may have fewer side effects as well."
Building on Momentum
In 2020, Reiman told Newsweek he was "agnostic" on whether amyloid played the key role in the development of the disease, which the Baptists have long argued. Now he says the success of the amyloid drugs suggests they may be right. The recent developments are "a big deal" and suggests "that we're on the right track."
Like Aisen, he hopes eventually to conduct preventative trials on cognitively unimpaired individuals at genetic risk for Alzheimer's disease, in whom even the most sensitive biomarker tests cannot detect plaques. In the long run, he notes, "amyloid should not be the only target by any stretch of the imagination." But he says the "the implications for prevention are extraordinary."

"There's no guarantee that the prevention therapies are going to work," he says. "But gosh, now we have the chance to find out, and it's been a long time coming. My hope is we begin to see more successful developments. And we'll do everything we can to find out which prevention therapies might work sooner later."
For his part, Bateman can only recall with wonder how much—and how quickly—things have changed. Bateman, who invented the first blood tests to be used clinically and is one of the field's leading authorities on biomarkers, recalls the mood several years ago at the Alzheimer's Association International Conference, one of the field's largest.
"That meeting was more like a funeral," he recalls. "The latest amyloid trials were failing. Nothing was working. People were hopeless. People were so just saddened. All this hope had been placed on being able to try to make a difference, and trial after trial after trial were showing absolutely nothing. It was just zeros across the board."
The Association's last meeting, in Amsterdam in July, was "celebratory," he says. The bars were packed and some clinicians were even high-fiving in the hallways. "People were so excited by the future and what's possible and what can be done and everything else," he says. "It's super, super exciting. It just doesn't compare with anything else. It's an incredible time. There's just no good analogy for it."
Many of those working to find a cure for the disease (though certainly not all) describe these recent events as "game changers," the most compelling evidence to date that the moribund field is finally on the right track.
"For all of history, people have been afflicted by senility and dementia, and for thousands of years, you couldn't do anything about it," says Bateman. "Even in recent history, you could only diagnose people after they died. Now a single blood sample can with 95 percent accuracy identify Alzheimer's. And in just six to 18 months, we can remove plaques from the brain that have built up over 20 years. We can for the first time change the course of how fast the disease progresses. To me, it's just absolutely incredible. It's really profound."











